UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File No.) | (IRS Employer Identification No.) |
|
|
||
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including
area code: (
N/A
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| The |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01. Other Events.
On January 5, 2023, the Company made available a Corporate Presentation on the Investor Relations page of the Company’s website, which will be used at investor and other meetings. A copy of the Corporate Presentation is attached hereto as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference. The Company does not undertake to update this presentation.
1
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Protara Therapeutics, Inc. Corporate Presentation, January 2023 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
2
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| PROTARA THERAPEUTICS, INC. | ||
| Date: January 5, 2023 | By: | /s/ Jesse Shefferman |
| Jesse Shefferman | ||
| Chief Executive Officer | ||
3
Exhibit 99.1

CORPORATE PRESENTATION January 2023

FORWARD LOOKING STATEMENTS 2 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Statements contained in this press release regarding matters that are not historical facts are "forward looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Protara may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “designed,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words or expressions referencing future events, conditions or circumstances that convey uncertainty of future events or outcomes to identify these forward - looking statements. Such forward - looking are not limited to, statements regarding Protara’s intentions, beliefs, projections, outlook, analyses or current expectations concerning, Protara’s business strategy, including its development plans for its product candidates and plans regarding the timing or outcome of trials; statements related to expectations regarding interactions with the FDA , Protara’s financial footing; statements regarding the of Protara’s product candidates; and Protara’s outlook for the remainder of the year. Because such statements are subject to risks and results may differ materially from those expressed or implied by such forward - looking statements. Factors that contribute to the uncertain looking statements include: risks that Protara’s financial guidance may not be as expected, as well as risks and uncertainties associated development programs, including the initiation and completion of non - clinical studies and clinical trials and the timing of required filings regulatory agencies; the impact of the COVID - 19 pandemic on Protara’s business and the global economy as well as the impact on organizations, study sites or other clinical partners; general market conditions; changes in the competitive landscape; changes in Protara’s commercial plans; Protara’s ability to obtain sufficient financing to fund its strategic plans and commercialization efforts; having to use other than expected; the impact of market volatility on cash reserves; the loss of key members of management; the impact of general U.S. economic, industry, market, regulatory or political conditions; and the risks and uncertainties associated with Protara’s business and general, including the risks and uncertainties described more fully under the caption “Risk Factors” and elsewhere in Protara's filings and States Securities and Exchange Commission. All forward - looking statements contained in this press release speak only as of the date on and are based on management's assumptions and estimates as of such date. Protara undertakes no obligation to update any forward - whether as a result of the receipt of new information, the occurrence of future events or otherwise, except as required by law.

ADVANCING TRANSFORMATIVE THERAPIES FOR PEOPLE WITH CANCER AND RARE DISEASES Applying modern scientific advancements to established mechanisms LEAD PROGRAM: TARA - 002 IN NON - MUSCLE INVASIVE BLADDER CANCER (NMIBC) AND LYMPHATIC MALFORMATIONS (LMS) Cell - based immunopotentiator based on originator therapy OK - 432, which is approved in multiple oncology indications and LMs in Japan NMIBC: Phase 1 clinical trial of TARA - 002 in adults with high - grade NMIBC - Phase 1a read out expected 1H 2023; preclinical work for I - O combos ongoing LMs: Phase 2 clinical trial of TARA - 002 expected to initiate in 2023; FDA granted Rare Pediatric Disease Designation MID - STAGE DEVELOPMENT PROGRAM PROVIDES DIVERSIFICATION & ADDITIONAL GROWTH POTENTIAL IV Choline in intestinal failure associated liver disease (IFALD) IFALD: Completed End of Phase 2 dialogue with FDA and aligned on Phase 3 clinical trial design ~25 team members who prioritize creativity, diverse perspectives and tenacity Solid balance sheet with cash runway into 2H 2024 as of September 30, 2022 3 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

PIPELINE ADDRESSES MULTIPLE INDICATIONS WITH HIGH UNMET NEED Pre - Clinical IND Cleared Phase 1 Phase 2 Phase 3 IMMUNOLOGY, ONCOLOGY TARA - 002 – Lyophilized, inactivated Group A Streptococcus Lymphatic Malformations (LMs)* Non - Muscle Invasive Bladder Cancer (NMIBC) TARA - 002 combinations HEPATOLOGY, GI, METABOLICS IV Choline Chloride for Injection – Phospholipid Substrate Replacement Intestinal Failure Associated Liver Disease (IFALD)** ,† *TARA - 002 Granted Rare Pediatric Disease Designation for the treatment of LMs. **Granted Orphan Drug and Fast Track Designations by the U.S. FDA † Phase 1 PK study to be conducted in addition to Phase 3 study 4 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002 Lyophilized, Inactivated Group A Streptococcus pyogenes

TARA - 002: CELL - BASED IMMUNOPOTENTIATOR WITH SIGNIFICANT POTENTIAL TARA - 002 is an investigational, genetically distinct strain of Streptococcus pyogenes that is inactivated while retaining its immune - stimulating properties TARA - 002 is manufactured under GMP conditions from the same Master Cell Bank as originator therapy OK - 432 (1) , which is approved for LMs and a number of oncology indications in Japan OK - 432 has been studied in many different types of cancer and there are close to 2,000 separate publications for OK - 432 listed in PubMed Protara has worldwide rights ex - Japan & Taiwan for TARA - 002/OK - 432 1. Marketed in Japan and Taiwan as Picibanil ® . Note: Manufacturing modifications reflect manufacturing to U.S. cGMP standards LIVE CELLS INACTIVATED 6 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002: MECHANISM OF ANTI - TUMOR/ ANTI - CYSTIC ACTIVITY Th1 Like Anti - Tumor Cytokine Response IL - 10 7 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute TNF - α IFN - γ IL - 2 IL - 6 IL - 8 Multi - Cytokine Inducer (1)(2)(3) IL - 12 GM - CSF G - CSF 1. Fujimoto T., et al. J Immunol. 1997: 5619. | 2. Ryoma Y, et al. Anticancer Res. 2004; 3295 - 3298. | 3. Zhao H, et al. Microbiol. Immunol. 1994; 183 - 190.

OK - 432: HUMAN EFFICACY DATA IN MULTIPLE INDICATIONS OK - 432 has been approved (ex - US) or studied in multiple indications • Ovarian cancer • Malignant mesothelioma • Pancreatic cancer • Esophageal cancer • Oral squamous cell cancer • Hepatocellular cancer • Ranula • Thyroglossal cysts • Pleurodesis • Seroma • Symptomatic lymphocele • Auricular hematoma APPROVED INDICATIONS IN JAPAN 1 Lymphangiomas (Lymphatic Malformations) • Gastric cancer combo with chemo (post - operative) • Primary lung cancer combo with chemo • Reduction of ascites in gastrointestinal cancer • Reduction of pleural effusion in lung cancer • Unresponsive head, neck & thyroid cancer 1. Full Prescribing Information. Chugai Pharmaceuticals. 2016 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute 8 OK - 432 CLINICAL RESEARCH CONDUCTED IN: Non - Muscle Invasive Bladder Cancer

TARA - 002 Non - Muscle Invasive Bladder Cancer (NMIBC)

TARA - 002 IN NMIBC: PROFILE SUPPORTS POTENTIAL CELL - BASED IMMUNOPOTENTIATOR WITH NOTABLE PATIENT EXPERIENCE Proven Anti - Cancer MOA • Elicits Th1 type response inducing multiple cytokines to produce an anti - tumor effect • Mechanistically similar to the current SOC, Bacille Calmette - Guérin (BCG) • Potential to work well in combination with other NMIBC therapeutics Manufacturing Advantages • State - of - the - art U.S. manufacturing facility • TARA - 002 manufacturing process supported by 40 years of production history of OK - 432 Modality Familiar with Physicians • MOA with which urologists are familiar and have been using for decades • Intravesical administration is preferred clinical approach among urologists (1) Promising Existing Clinical Data • ~150 NMIBC patients tested with OK - 432 demonstrated promising results • Treatment generally well tolerated 1. Market Research Conducted by Protara Therapeutics | Note: OK - 432 is not approved for NMIBC 10 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002 IN NMIBC: CLINICAL EVIDENCE FROM PREDECESSOR THERAPY OK - 432 PROVIDES STRONG RATIONALE FOR DEVELOPMENT IN NMIBC Data across multiple studies in ~150 NMIBC patients treated intravesically shows that OK - 432: • Was generally well - tolerated, with safety and tolerability observed across a range of doses • Demonstrated treatment effect and lower rates of recurrence vs. control group, including in the randomized, controlled setting Fujita K, et al. Cancer. 1987; 59: 2027 - 2030 | Fujita K, et al. Cancer Detection and Prevention. 1988; 11: 397 - 403 | Sun X, et al. China Journal of Medicine. 2004; 14: 49 - 54 | Liu, Z.H, et al. Oncology Letters. 2017;13:4818 - 4824. | Fujioka et al. Acta Urol. Japan. 1989; 35: 253 - 257 OK - 432 Study Dose Regimen Total Pts/ OK - 432 Pts OK - 432 Efficacy Results Fujita, 1987 Bladder Cancer 2 to 5 KE intratumoral, 5 KE intravesical instillation 78 / 37 In previously unresected tumors, 5 recurrences in OK - 432 treated patients vs. 12 recurrences in the control arm (p<0.05) at 36 months. For patients with primary disease, OK - 432 showed a benefit over control in multiple subgroups (multifocal, sessile, or high grade). Fujita, 1988 Bladder Cancer 2 to 5 KE intratumoral, 5 KE intravesical instillation 36 /17 OK - 432 reduced recurrence rates of disease ( 35% recurrence in OK - 432 group compared to ~73% recurrence in surgery alone group ); OK - 432 caused lymphocyte infiltration into carcinomas (as evidenced by histology after resection). Sun and Qiu, 2004 Bladder Cancer 3 KE intravesical instillation weekly for 6 weeks then monthly for 6 months 30 / 30 At a mean follow - up of 14 months , tumor recurrence was in 16.6% of patients, with no recurrence in 83.4% of patients . OK - stimulated secretion of IL - 2 and TNF α (p<0.05 for both). Liu et al., 2017 NMIBC 3 KE (in 30 ml) intravesical instillation 55 / 55 Overall, patients treated in the study had a recurrence rate of 34.5% and progression rate of 10.9%. Treatment with OK - 432 was more effective when patients were negative for PD - L1 ( 16.7% 4.2% progression rate ), regardless of disease stage/grade. Fujioka et al., 1989 NMIBC 5 KE (intravesical), 10 KE (intratumoral) 38 / 38 Tumors were eliminated endoscopically in 6 of 28 (21.4%) which OK - 432 was intravesically instilled [Stage Ta = 5 patients, T1 = 1 patient; all patients Grade 1], and 3 of 10 (30%) patients with intratumoral OK - 432 injection. 11 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002 IN NMIBC: TARGET PATIENT POPULATION NMIBC is categorized and treated based on risk stratification, determined by combination of tumor grade, stage, size, recurrence history and focality Patients diagnosed with bladder cancer annually 1 Patients with NMIBC 2 Initial target patient population: Patients with high grade NMIBC who are candidates for immunotherapy 1 - 3 80,000 65,000 ~30,000 Treatment goal is to prevent: 1. Progression 2. Cystectomy 3. Recurrence 1. National Cancer Institute, SEER Cancer Stat Facts: Bladder Cancer, 2021. | 2. Anastasiadis et al. Therapeutic Advances in Urology, 2012. | 3. Market Research Conducted by Protara Therapeutics NMIBC, non - muscle invasive bladder cancer. © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute 12

TARA - 002 IN NMIBC: PHASE 1 CLINICAL TRIAL DESIGN PHASE 1 Dose finding, open - label trial with expansion evaluating intravesical TARA - 002 in adults with high - grade NMIBC Phase 1a: Dose Escalation 1 Phase 1b: Expansion Cohort 1 3+3 Dose Escalation Design Enroll 10 KE 20 KE 40 KE NMIBC HGCIS or HGTa in patients previously treated or unable to access BCG (BCG - naïve 2 ) N = 9 – 18 patients • Evaluate safety, tolerability and preliminary signs of anti - tumor activity of TARA - 002 and establish MTD and RP2D for Phase 2 study Enroll RP2D NMIBC HGCIS active disease 3 N = 12 patients * *Subjects enrolled in the dose expansion phase will not include subjects previously enrolled and treated in the dose escalation phase • Further assess safety and preliminary signs of anti - tumor activity of TARA - 002 at the established RP2D 1. Subjects will receive weekly intravesical doses of TARA - 002 instillation for 6 weeks | 2. Defined as not previously treated with or unable to access BCG. | 3. Defined as disease present at last cystoscopic evaluation during the dose expansion phase. Definitions: BCG, bacillus Calmette - Guérin; HGCIS, high - grade carcinoma in situ; HGTa, high - grade Ta; KE, Klinische Einheit; MTD, maximum tolerated dose; RP2D, recommended phase 2 dose; TURBT, trans urethral resection of bladder tumor. © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute 13

TARA - 002 Lymphatic Malformations (LMs)

TARA - 002 IN LMs LYMPHATIC MALFORMATIONS Rare, non - malignant lesions consisting of dilated, lymphatic fluid - filled sacs caused by abnormal development of the lymphatic endothelial system (1) EPIDEMIOLOGY Epidemiology: incidence of lymphatic malformations is ≈1,400 - 1,800 LM cases per year (2) CURRENT TREATMENT OPTIONS Current treatment options include surgical excision with high complication (33%) and recurrence (55%) rates (3) as well as off - label use of sclerosants FDA GRANTED PEDIATRIC RARE DISEASE DESIGNATION Majority of LMs present at birth (65 - 75%) or by age 3 (80 - 90%) during active lymphatic growth period (3) 1. Brouillard P, et al. J Clin Invest. 2014;124:898 - 904. | 2. Internal company estimates | 3. Ha J, et al. Curr Ped Rev. 2014;10:238 - 248. 15 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002 IN LMs: CLEAR EVIDENCE OF BIOLOGIC ACTIVITY OBSERVED WITH PREDECESSOR THERAPY OK - 432 Completed clinical study of OK - 432 (TARA - 002 predecessor therapy) in U.S. suggests effectiveness with strong support for safety profile Protara Therapeutics data on file BEFORE AFTER BEFORE AFTER BEFORE AFTER BEFORE 16 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute AFTER

TARA - 002 IN LMs: ROBUST RESULTS OF COMPLETED CLINICAL TRIAL (1) IN U.S. OBSERVED WITH PREDECESSOR THERAPY OK - 432 69% CLINICAL SUCCESS ǂ IN IMMEDIATE TREATMENT GROUP 6 MONTHS AFTER ENROLLMENT 84% * CLINICAL SUCCESS ǂ IN PATIENTS WITH MACROCYSTIC LESION TYPES 69% 7.5% Immediate Treatment Group (N=110) Delayed Treatment Group Pre - Treatment** (N=40) ITT: Observations 6 Months After Enrollment 62% 28% 0% 22% 32% Macrocystic Lymphangiomas (n=77) Mixed (n=47) Microcystic Lymphangiomas (n=14) Complete Response Substantial Response • Patients with radiographically confirmed macrocystic lesions had the greatest chance for clinical success • In those patients with mixed lesions, clinical success was still achieved Complete or Substantial Response by Radiographically Confirmed Lesion Type** 84% • During this same period, 7.5% of patients in the delayed treatment group experienced spontaneous regression of LM • Treatment: 1 - 4 injections at 8 - week intervals max of 0.2mg/session (2KE) P < 0.0001 60% ǂ Clinical Success was defined as complete or substantial response *Reflects data prior to dosing with OK - 432. After dosing, the clinical success rate was 66%, which was not statistically different from the Immediate Treatment Group **Results were analyzed by lesion type across all treatment groups 1. Results based on retrospective analysis of source verified data that included the full dataset of subjects enrolled in randomized study between January 1998 and August 2005, including data in the published study (Smith et al. 2009) which included subjects enrolled between January 1998 and November 2004. 17 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002 IN LMs: COMPELLING SAFETY RECORD OF PREDECESSOR THERAPY OK - 432 Most common AEs with treatment were local injection site reactions, fever, fatigue, decreased appetite, with resolution within two weeks Treatment emergent SAEs related to OK - 432: reported in 4.1% of patients, with the most severe events being airway obstruction and facial paralysis due to swelling post - injection that required tracheostomy and hospitalization. Both of these events were reported as resolved One SAE related to OK - 432 led to discontinuation: Proptosis of the eye One SAE not related to OK - 432 led to death: Death due to tracheostomy tube obstruction Safety Profile* *Results based on retrospective analysis of source verified data that included the full dataset of subjects enrolled in randomized study between January 1998 and August 2005, including data in the published study (Smith et al. 2009) which included subjects enrolled between January 1998 and November 2004. 18 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute
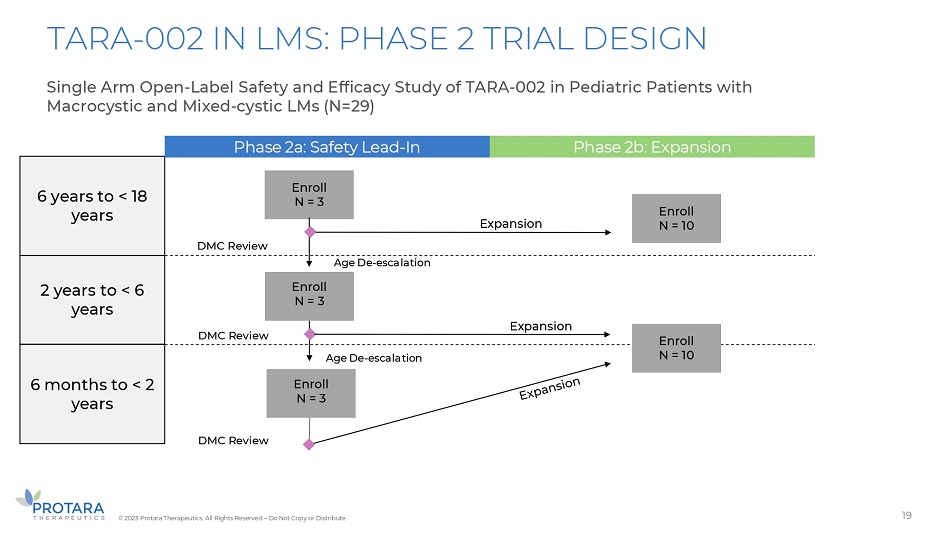
TARA - 002 IN LMS: PHASE 2 TRIAL DESIGN Single Arm Open - Label Safety and Efficacy Study of TARA - 002 in Pediatric Patients with Macrocystic and Mixed - cystic LMs (N=29) 6 years to < 18 years 2 years to < 6 years 6 months to < 2 years Phase 2a: Safety Lead - In Phase 2b: Expansion Enroll N = 3 Enroll N = 3 Expansion Age De - escalation Age De - escalation Expansion DMC Review DMC Review DMC Review Enroll N = 3 Enroll N = 10 Enroll N = 10 19 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

IV CHOLINE CHLORIDE Intestinal Failure Associated Liver Disease (IFALD)

IV CHOLINE IN IFALD: LATE - STAGE OPPORTUNITY FOR AN UNMET MEDICAL NEED 21 1. Buchman A, et al. JPEN. 2001;5:260 - 268. IFALD, intestinal failure associated liver disease; IV, intravenous; PK, pharmacokinetic; PN, parenteral nutrition. © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute HIGH UNMET NEED AMONG PATIENTS • Patients dependent on PN cannot absorb sufficient levels of choline. Data confirms that choline deficient diets results in steatosis and cholestasis. 1 There are currently no approved PN treatments that offer sufficient choline CLINICAL HISTORY SUPPORTING CHOLINE SUBSTRATE REPLACEMENT IN PATIENTS WITH IFALD • A Phase 2 study demonstrated the clinical potential of choline substrate replacement therapy by reversing certain hallmark pathologies of IFALD 1 INTELLECTUAL PROPERTY RECENTLY SECURED • Patent and Trademark Office issued to the Company a patent claiming a sterile aqueous choline salt composition with a term expiring in 2041 CLEAR REGULATORY AND CLINICAL PATH FORWARD • Received Orphan Drug and Fast Track Designations from FDA. Positive End of Phase 2 meeting with FDA requesting Phase 1 PK study and Phase 3 clinical trial to complete registrational package

IV CHOLINE IN IFALD: PHASE 2 TRIAL RESULTS 22 Improvement in Steatosis and Cholestasis CLINICALLY MEANINGFUL IMPROVEMENT IN STEATOSIS CHOLESTASIS IMPROVEMENT: ALL PATIENTS *(1) At Baseline After 24 Weeks *Mixed model for repeated measurement (MMRM) method used ǂ A placebo subject was excluded from all analyses due to likely IV contrast - induced imaging abnormalities, confirmed by independent radiologist n=14 ǂ (7 active; 7pbo) p=0,0764 p=0,0565 p=0,0081 p=0,0050 p=0,0069 p=0,0052 p=0,0016 400 350 300 250 200 150 100 p=0,6500 50 0 - 50 - 100 - 150 - 200 2 4 6 12 16 20 24 34 Assessment Week Choline D/Cd Choline Chloride Placebo LS Means (+/ - std err) of change from Baseline of ALP (U/L) // // 1. Protara Therapeutics re - analysis of patient CRFs, data on file © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

SUMMARY

2023: FOCUSED EXECUTION DESIGNED TO POSITION PROTARA FOR LONG - TERM GROWTH STRONG BALANCE SHEET: $107M of cash, cash equivalents and investments as of September 30, 2022 expected to fund operations into 2H 2024 19.3M COMMON SHARE EQUIVALENTS: 11.3M Common + 8.0M Preferred on as converted basis as of September 30, 2022 24 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Complete Phase 1a Portion of NMIBC Clinical Trial and Initiate Phase 1b Initiate Phase 2 Clinical Trial in LMs Complete Prospective Prevalence Study to Refine Development Pathway for IV Choline Disciplined Approach to Investment

APPENDIX

High rate of recurrence with 3 - year rate estimated at up to 80% (3) NMIBC REPRESENTS THE MOST COMMON FORM OF BLADDER CANCER Bladder Cancer in the U.S. most prevalent cancer in the U.S. 6 th 4x more likely to be diagnosed in men (2) 9 in 10 are age 55+ (1) NMIBC makes up ~80% of all bladder cancer with ~65,000 diagnosed per year in the U.S. (4) NMIBC patients are treated by a urologist Significant increase in recurrence, progression & an escalated number of patients needing cystectomies (5) (1) 1. National Cancer Institute. SEER Bladder Cancer – Stat Facts. Accessed February 5, 2021. | 2. Saginala, K. et al. Med Sci. 2020. | 3. Campbell Walsh 11th edition, Elsevier. | 4. Anastasiadis et al. Therapeutic Advances in Urology, 2012. | 5. Ourfali, S. et. al. European urology focus, 2019. 26 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

CURRENT STANDARD OF CARE HIGHLIGHTS HIGH UNMET NEED FOR PATIENTS Initial Presentation Recurrence Repeat TURBT Cystectomy Bladder Removal Intravesical Agents Experimental Agents in Development IV Treatment Transfer to Medical Oncologist • Checkpoint inhibitors Present to PCP : • Blood in urine • Bladder irritation Urologist tests: • CT scan • Cytology • Cystoscopy Diagnose, Stage & Grade Prescribe BCG Up to 8/10 recur in 3 years 1 UNDER UROLOGIST CARE High Grade Referral & Testing TURBT Treatment Primary care / PCP Urologist Medical Oncologist 1. Campbell Walsh 11th edition, Elsevier. 27 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

ONGOING BCG SHORTAGE PRESENTS OPPORTUNITY FOR ALTERNATIVE TREATMENT OPTIONS AUA GUIDELINES ON BCG SHORTAGE BCG use has been limited to high - risk disease; induction to be prioritized over maintenance MERCK BECOMES SOLE MANUFACTURER Sanofi facility for Connaught strain of BCG goes offline; Merck’s TICE strain becomes only remaining option FDA WORKSHOP ON NMIBC Physicians report negative impacts on patient outcomes and trial recruitment due to BCG shortage FDA APPROVAL OF BCG BCG is approved for NMIBC with carcinoma in situ and high - risk Ta/T1 1. FDA workshop on BCG shortage ( 2021 ) 56% physicians still prefer giving BCG over chemotherapy to intermediate risk patients 1 92% physicians consider developing an alternative to BCG as first line therapy for high - risk NMIBC a priority 1990 2012 2019 2021 28 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

IV CHOLINE IN IFALD: PREVALENCE STUDY 29 © 2023 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute PREVALENCE STUDY TO ENHANCE UNDERSTANDING OF THE PATIENT POPULATION DESIGN Retrospective, observational study of patients in both academic & community settings POPULATION Patients dependent on PN for 6 or more months OBJECTIVE • Measure serum alkaline phosphatase (ALP) levels greater than 1.5 times the upper limit of normal (ULN) as a key marker of cholestasis RESULTS • ~ 31 % of all patients, irrespective of baseline levels, presented with ALP levels greater than 1 . 5 times the ULN at any given time during 6 to 36 months . • ~ 28 % of all patients had persistent ALP elevations greater than 1 . 5 times the ULN at 36 months . • At baseline, ~ 23 % of patients presented with ALP levels greater than 1 . 5 times the ULN with ~ 76 % presenting with greater than 1 . 5 times the ULN at any given time during 6 to 36 months and ~ 59 % with persistent ALP elevations greater than 1 . 5 times the ULN at 36 months . • Results support further exploration in patient population to determine rates of choline deficiency & steatosis . NEXT STEPS Prospective observational study under way to further characterize the prevalence of choline deficiency, as well as cholestasis and steatosis
